Connected. Intelligent. Automated.

Automation in Action
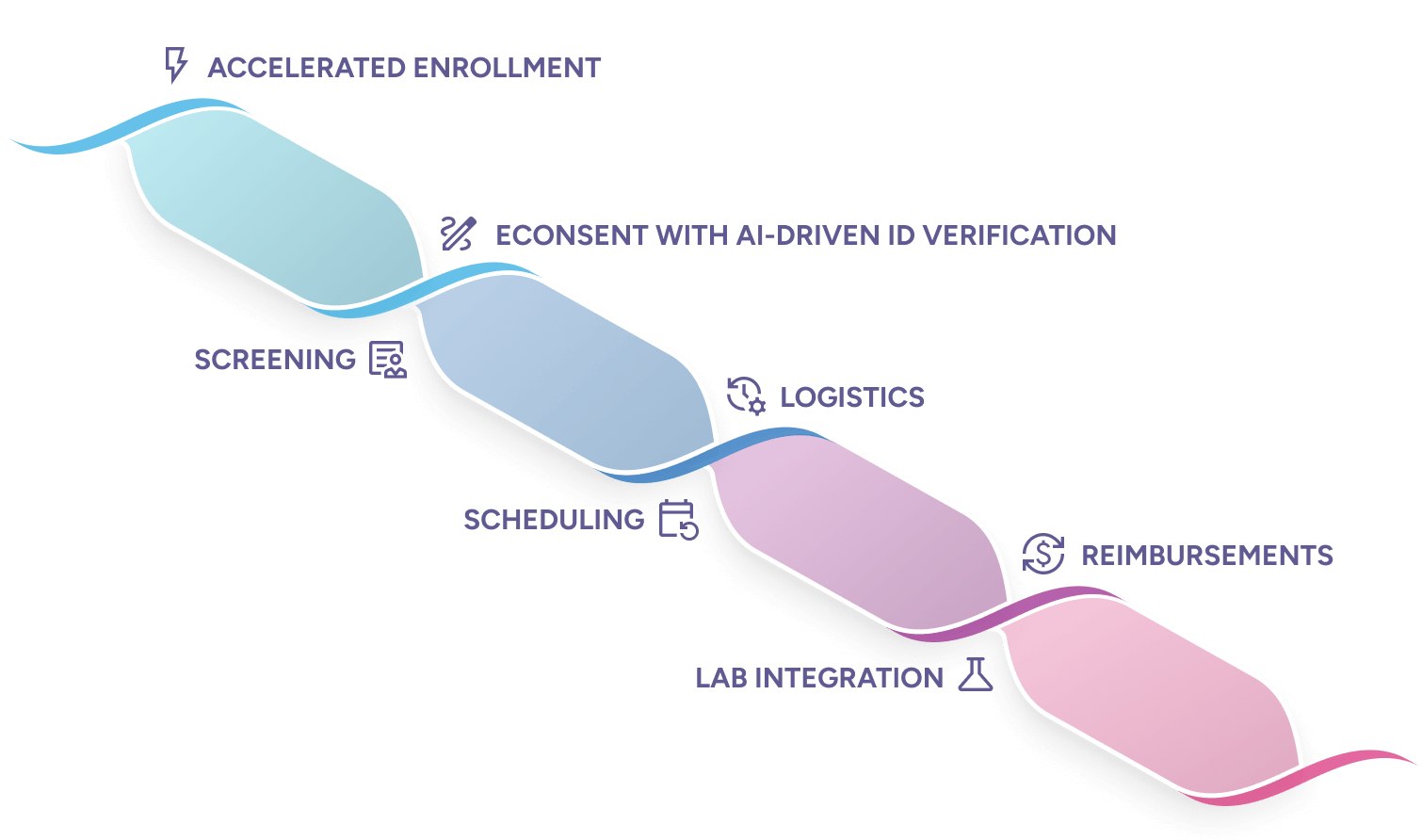
Accelerated Enrollment
Alethium’s fully automated enrollment streamlines every step, from recruitment and eligibility to account creation and eConsent. It enables 24/7 enrollment, accelerates first visits, and virtually eliminates protocol deviations.
Screening
Our CDP transforms inclusion and exclusion criteria into automated rules. Pre-screening questionnaires and structured data capture ensure consistency, create audit-ready records, and enable faster, more confident enrollment decisions.
eConsent with AI-driven ID verification
eConsent with AI-driven ID verification eliminates the cost, time, and risk of manual consenting. Participants can securely consent anywhere, anytime, while automated verification ensures 21 CFR Part 11 compliance and provides the flexibility needed for even the most complex global consent workflows.
Scheduling
Automated scheduling lets participants easily book visits, onsite care, and other study activities, all within protocol-defined windows. This streamlines operations, reduces administrative workload, and keeps participants on track, improving adherence and accelerating study timelines.
Logistics
Powered by an event-driven architecture, Alethium automates shipping, pickups, and other logistics, transforming complex supply operations into smooth, protocol-compliant workflows that save time and reduce errors.
Lab Integration
Alethium automates lab data exchange, capturing and verifying results in real time. This reduces manual handling, eliminates delays, and ensures complete data integrity.
Reimbursements
With event-driven automation, Alethium delivers validated reimbursement triggers directly to accounting systems, enabling rapid, compliant, and accurate disbursements across every study site and participant.
Additional Automations
Alethium connects seamlessly to preferred vendors and third-party systems, enabling tailored integrations that bring end-to-end automation to every study-specific workflow.
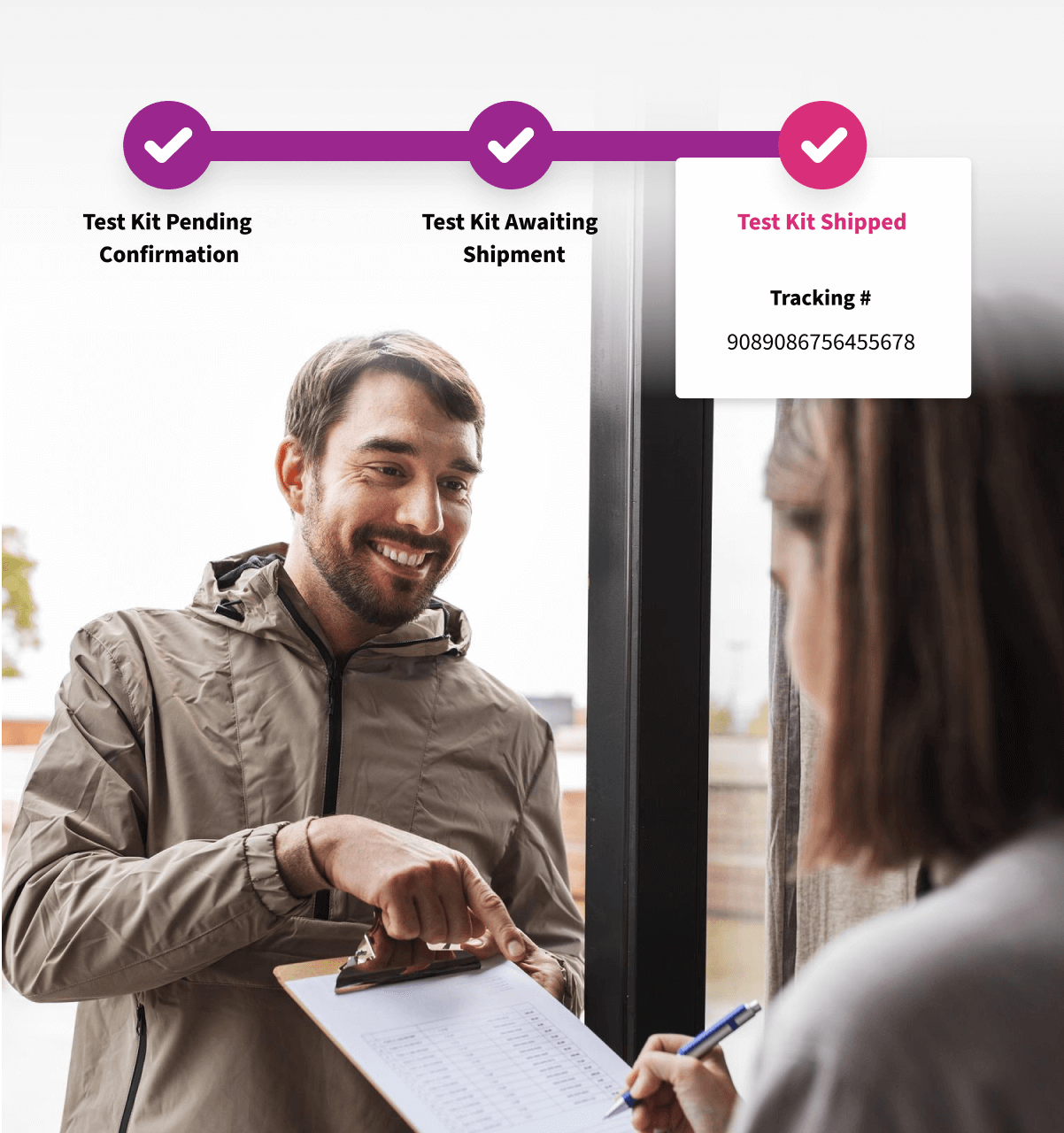
Event-Driven Automation
Alethium combines Behavior-Driven Development (BDD) with an event-driven architecture to deliver every eCOA, televisit, shipment, and service exactly on schedule. Protocol rules are enforced automatically, and trial events trigger instant actions, reducing site burden, protecting data integrity, and keeping every process aligned with study requirements.
Deliverables:
Validation rules and release criteria for consent-to-ship
Chain-of-custody configurations and milestone tracking
Event-driven shipping trigger definitions
Exception handling playbooks and escalation workflows
Comprehensive audit log mappings for shipping, receipt, and returns
Strategic Benefits
Deliver measurable efficiency, stronger compliance, and lower costs by automating and unifying clinical operations.
Deviation Prevention
The Automation Engine enforces protocol adherence, removes error-prone manual steps, and dramatically reduces the likelihood of protocol deviations.
Simplified Complexity
Alethium converts automation into real savings. Our event-driven platform brings Sponsors and CROs together on a single, automated system that streamlines workflows, cuts time and overhead while accelerating clinical trial delivery.
Lower Operational Cost
By automating core trial workflows and removing manual touchpoints, we shorten study timelines, cut monitoring and administrative expenses, and increase trial throughput. The net effect is faster results, more efficient programs, and a clear uplift in ROI.
Full Traceability & Reduced Risk
A continuous, event-driven audit log records every action as it occurs, producing an immutable, time-stamped trail that supports compliance, improves traceability, and reduces risk.
Behavior-Driven Automation
Behavior-Driven Development powers automated, risk-aware workflows that deliver transparency and stakeholder confidence. Automated processes ensure consistency, compliance, and participant safety at every step.
Schedule Your Personalized Demo
We listen to your priorities and focus on the risks that matter most to you. Every demo is tailored to your challenges.
FAQs - Common Questions
Embedded within Alethium’s CDP, the Automation Engine streamlines recruitment, eligibility, consent, logistics, labs, and reimbursements by applying protocol rules built with Behavior-Driven Development (BDD). Every automated action is captured in real time in Alethium’s event-driven audit log, ensuring full transparency, control, and confidence across your study operations.
The Automation Engine seamlessly manages digital recruitment, automated eligibility, intelligent eConsent, next-day temperature-controlled logistics, televisits, lab integration, and reimbursements. Protocol-driven triggers execute each process, capturing every action in real time in the event-driven audit log. The outcome: accelerated study startup, reduced site burden, lower costs, and reliable compliance across all trial activities.
All records are encrypted at rest with unique keys, and access is restricted by role-based permissions. When a participant withdraws or a study ends, keys are deleted, rendering residual data completely inaccessible. These safeguards maintain participant privacy, support audit readiness, and ensure full compliance with 21 CFR Part 11, HIPAA, and GDPR.
By automating protocol rules through Behavior-Driven Development (BDD), the Automation Engine ensures participants progress only when eligibility, consent, and timing criteria are satisfied. This removes manual errors, prevents off-schedule activities, and keeps all operations fully aligned with study protocols, minimizing deviation risk and delivering higher-quality data from day one.
From participant screening to study close, every automated action is logged instantly in Alethium’s event-driven audit log. Automation strictly follows verified protocol rules, providing a real-time, fully traceable record of system activity. Sponsors and CROs can rely on a living, accurate record of compliance, oversight, and operational performance.
Alethium’s Automation Engine accelerates First Patient In (FPI), lowers operational costs, improves data quality, and reduces participant withdrawals. Its automated workflows sustain engagement in hybrid and decentralized trials, while the real-time audit log ensures full CDP compliance.

