eCOAs that Meet Complex Trial Demands
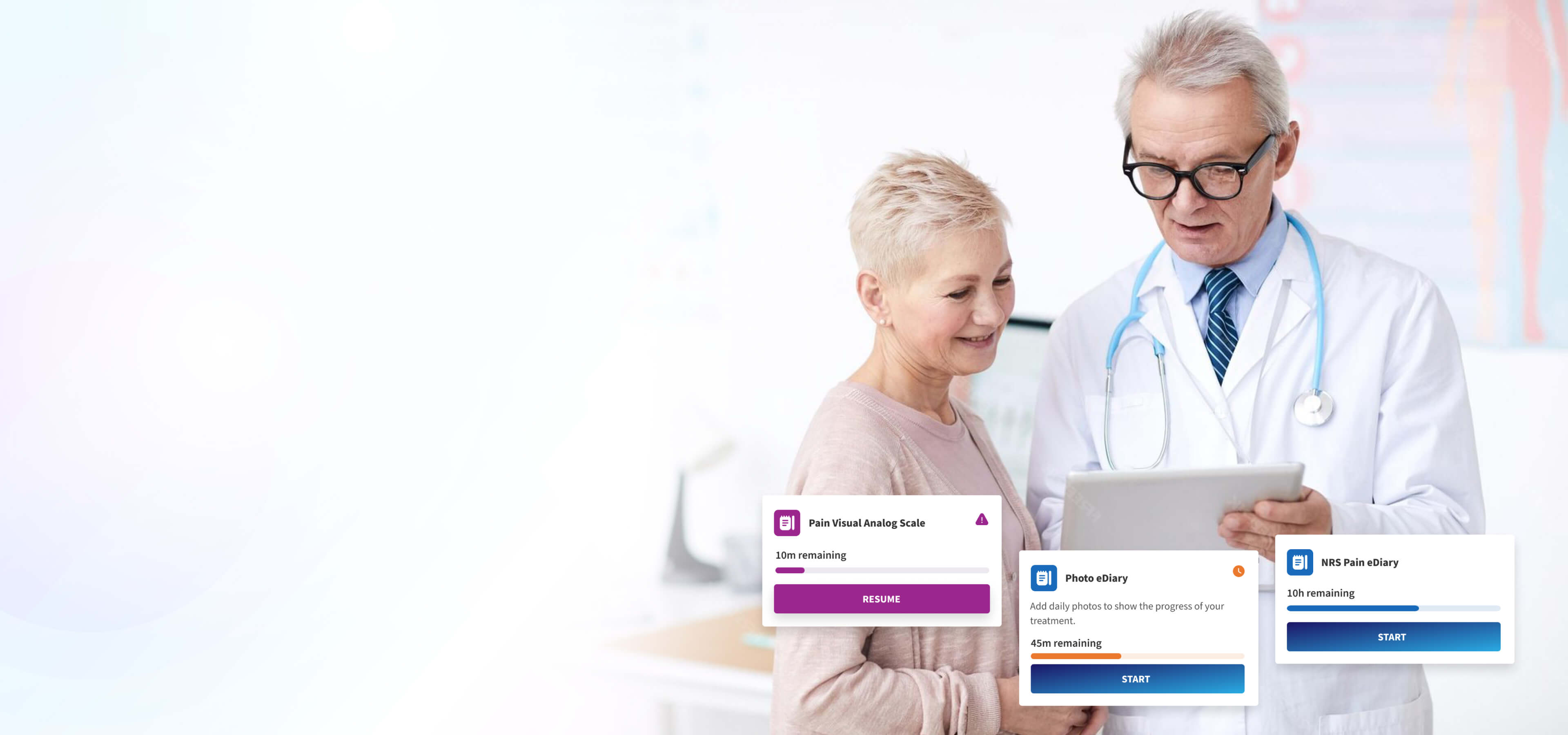
Scoring-Based eCOA
Alethium calculates even the most complex scoring-based clinical assessments with precision. Designed to support advanced instruments such as the Schizophrenia Cognition Rating Scale (SCoRS), Alzheimer’s Disease Composite Score (ADCOMS), and Multiple Sclerosis Functional Composite (MSFC). Each response is validated, scored in real time, captured in the event-driven audit trail and immediately available for analysis.
Photo and Source Document Capture
All required photographic and document-based evidence is captured according to protocol and instantly connected to the participant record for complete traceability.
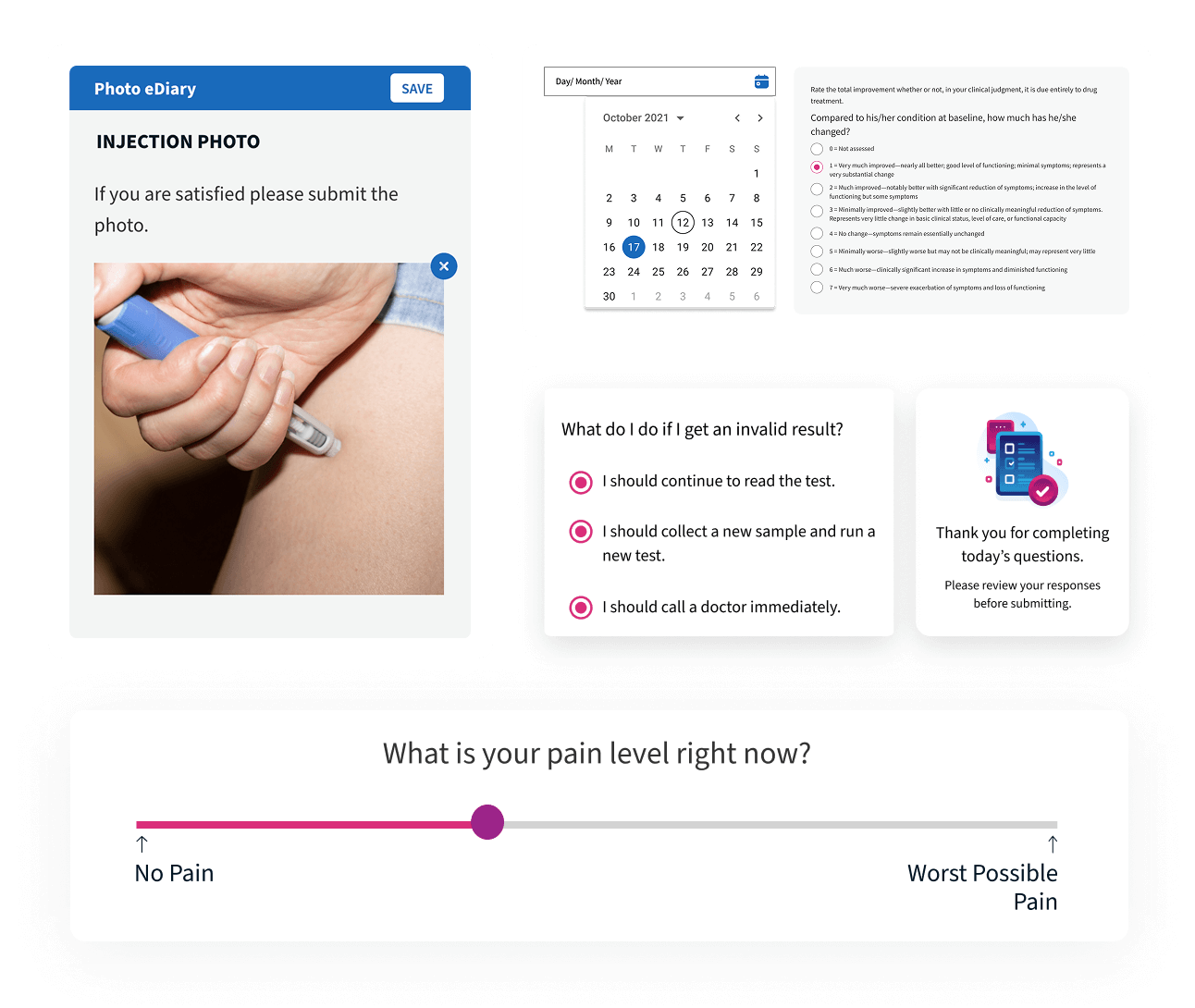

Point-of-Care PROs
Point-of-care ePROs allow participants to complete assessments directly at the site on any device, with automatic linking to the participant record and full audit logging for compliance and security.
Licensed ePROs
Alethium centralizes licensed instrument management, streamlining negotiation, procurement, integration, and version control within a single compliant system.

Digital-First Benefits
Built for Complexity
Alethium’s event-driven architecture aligns with Risk-Based Quality Management (RBQM) to ensure eCOA are delivered exactly as the protocol defines. Each trigger, based on cohort, visit window, dosing milestone, re-consent, or safety event, is logged, verified, and traceable within the audit trail.
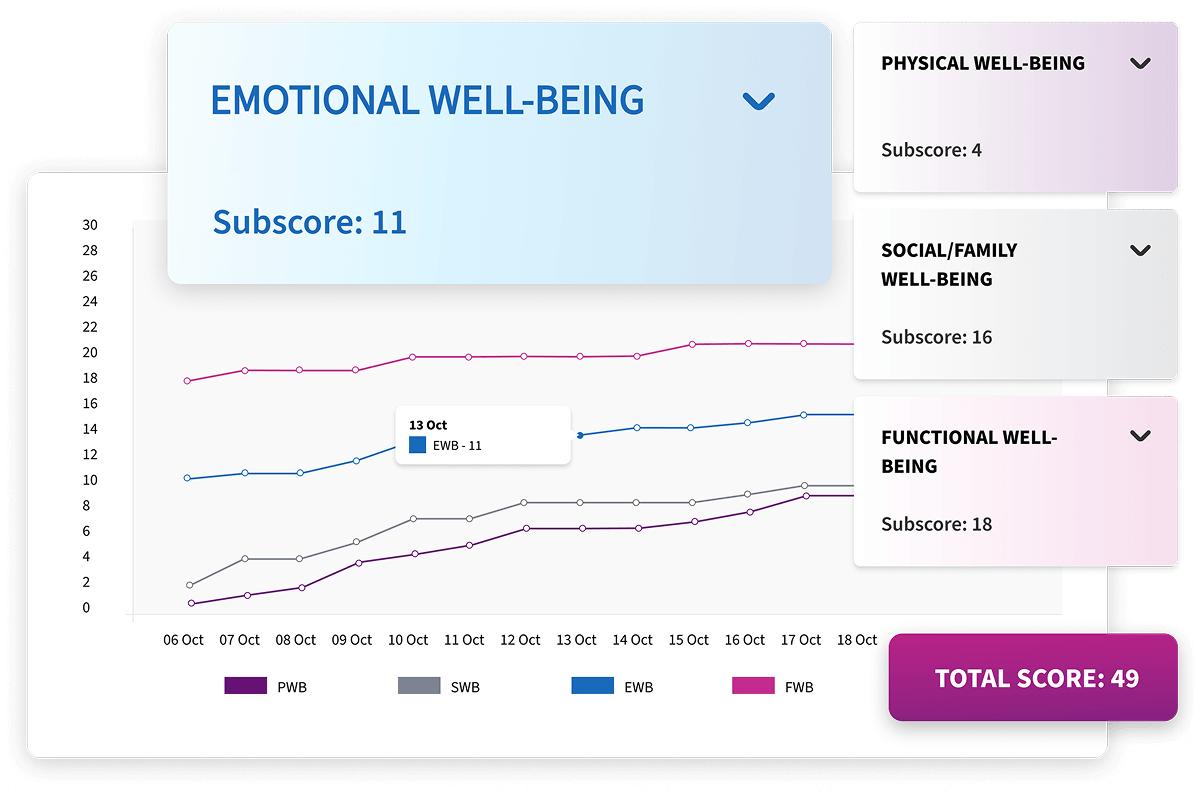
Longitudinal Reports
Alethium connects visit data across participants and timepoints to reveal meaningful trends, emerging risks, and protocol deviations. Interactive visualizations update instantly, allowing clinical teams to act quickly, maintain oversight, and ensure ongoing data integrity throughout the study lifecycle.
Responsive Design
A responsive interface adapts seamlessly across mobile, tablet, and desktop devices, maintaining full functionality and intuitive navigation so participants, investigators, and monitors can complete tasks effortlessly wherever they are.
Event-Driven Audit Log
The event-driven audit log maintains complete data integrity by recording every action in real time, creating an immutable record that supports Risk-Based Quality Management (RBQM), ensures compliance, and enables truly actionable analytics throughout the study lifecycle.
Data Security
Our CDP' infrastructure is fully encrypted in transit and at rest, maintaining compliance with SOC 2, HIPAA, GDPR, 21 CFR Part 11, and LGPD standards, with regional hosting options available across the United States, European Union, and Brazil for secure, jurisdictional data management.
Global Reach
Our platform supports ClinRO, ObsRO, ePRO, and eDiary instruments in more than 400 languages, including multi-byte and right-to-left scripts, ensuring global accessibility and consistent data quality across diverse participant populations.
Behavior-Driven Development (BDD)
Alethium’s event-driven architecture and Behavior-Driven Development (BDD) ensure the exact ePRO, ClinRO, ObsRO, or eDiary is delivered for the most complex protocol requirements.
How it's done:



Schedule Your Personalized Demo
We listen to your priorities and focus on the risks that matter most to you. Every demo is tailored to your challenges.
FAQs - Common Questions
Alethium’s digital-first eCOA automates scoring for even the most advanced instruments, including the Schizophrenia Cognition Rating Scale (SCoRS), Alzheimer’s Disease Composite Score (ADCOMS), and Multiple Sclerosis Functional Composite (MSFC). Responses are validated and scored instantly through Behavior-Driven Development (BDD) logic, ensuring accuracy, protocol adherence, and a complete audit-ready record—without manual scoring or reconciliation.
Alethium’s digital-first approach means every eCOA workflow is designed natively for automation and integration, not adapted from paper-based processes. From scheduling to evidence capture, each task is driven by protocol-defined logic within Alethium’s CDP. This eliminates manual handoffs, accelerates data flow, and ensures assessments are consistent, compliant, and audit-ready.
Alethium’s eCOA platform enables Point-of-Care ePROs and eDiaries that participants can complete securely on any device. Each session is logged automatically, minimizing site burden, improving participant adherence, and eliminating manual reconciliation between paper and electronic records.
Yes. Alethium provides multilingual eCOA experiences and regional hosting in the US, EU, and Brazil. This ensures HIPAA, GDPR, and LGPD compliance, making it suitable for global studies.
The solution reduces deviations, accelerates data capture, improves participant convenience, and provides audit-ready traceability. It combines Behavior-Driven Development-driven (BDD) workflows with device-agnostic access and integrated instrument management.
BDD expresses complex protocol rules in plain language and translates them into automated workflows. This guarantees every ePRO, ClinRO, ObsRO, and eDiary is delivered exactly as defined—on time, every time—reducing human error, ensuring compliance, and improving data precision across all study sites.
Every eCOA entry is recorded as an immutable event, providing real-time traceability and instant visibility for auditors, CROs, and Sponsors. The audit log verifies that every action—from data entry to system-triggered alerts—is captured, validated, and compliant with global regulatory standards.
Every eCOA entry is recorded as an immutable event, providing real-time traceability and instant visibility for auditors, CROs, and Sponsors. The audit log verifies that every action—from data entry to system-triggered alerts—is captured, validated, and compliant with global regulatory standards.
Every entry is written instantly to the event-driven audit log, encrypted at rest, and tied to the participant record. With role-based access, electronic signatures, and secure version control, Alethium’s eCOA is fully 21 CFR Part 11 compliant.




