Instant. Accurate. Actionable Data.
Alethium Clinical Data Platform (CDP) turns trial data into actionable insights. All data are sourced directly from the event-driven architecture and audit log, ensuring that every report and custom dashboards reflects verified real-time study data.

PII Protection
Each participant record is encrypted with a unique key, protecting all PII/PHI at the database level. Role-based access controls and automated export sanitization prevent unauthorized access or leakage of sensitive data.
Event-Driven Architecture
The analytics engine sources data directly from the event-driven audit log, ensuring that all metrics, dashboards, and reports are derived from validated operational events. This architecture maintains data provenance, supports regulatory traceability, and guarantees alignment between analytical outputs and recorded study activity.
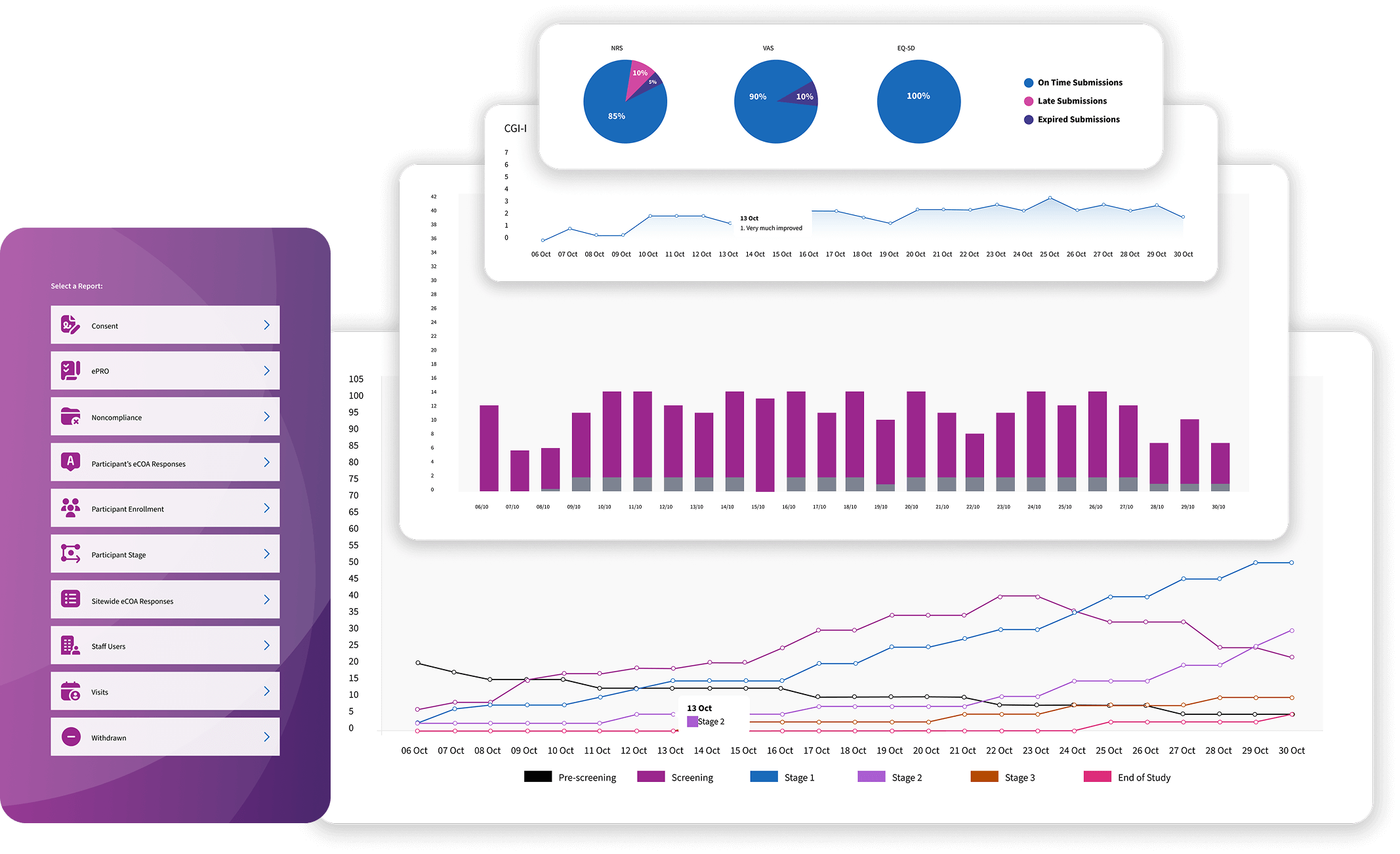
Longitudinal Reports
Our analytics operate within an RBQM framework, continuously evaluating longitudinal data to detect risk signals, assess operational changes, and trigger timely mitigation.
Custom Dashboards
Study-defined clinical trial dashboards surface study-specific KPIs, segmented by study, site, cohort, and timeframe to support precise performance monitoring.
Data Exports
Our standards-compliant data exports preserve schema integrity and are optimized for interoperability with advanced analytics environments, enabling continuous RBQM-driven monitoring and analysis.
KPI Configuration
Dashboards surface protocol-aligned, study-specific KPIs derived directly from validated data streams, enabling precise, real-time visibility into trial performance.
Least-Privilege Access
Role-based access controls (RBAC) enforce least-privilege data visibility, ensuring only authorized users can view sensitive data. Every access event is captured in the audit log, maintaining full traceability and compliance.
Strategic Insights Delivered
Built on Alethium’s event-driven audit log, advanced analytics provides real-time, validated insights that align with every recorded event. Risk indicators drive timely intervention, and end-to-end encryption with role-based permissions preserves data integrity throughout analysis and decision-making.
Schedule Your Personalized Demo
We listen to your priorities and focus on the risks that matter most to you. Every demo is tailored to your challenges.
FAQs - Common Questions
Alethium’s Advanced Analytics is built directly on an event-driven audit log, ensuring every metric reflects verified study activity. Unlike traditional dashboards, analytics are sourced from immutable data records, providing real-time clarity, complete traceability, and instant insight into trial performance, risk mitigation, and compliance across global studies.
Yes. Alethium’s KPI dashboards are study-defined, configurable, and aligned with protocol, surfacing the most critical insights for each trial.
Yes. Alethium’s analytics exports are structured for compatibility with advanced data visualization and statistical tools. Standards-based data exports ensure seamless integration with third-party systems while preserving encryption, version control, and validation traceability across all connected platforms.
Alethium’s analytics platform uses per-record encryption and least-privilege access to ensure that personally identifiable information (PII) remains fully secure. All exports exclude PII, and every data view is permission-controlled, logged, and encrypted — maintaining HIPAA, GDPR, and 21 CFR Part 11 compliance while protecting participant confidentiality.
Every analytic is event-driven and audit-ready, with role-based access, encryption, and alignment with 21 CFR Part 11, HIPAA, and GDPR.
Study-defined dashboards highlight protocol-specific KPIs, longitudinal performance trends, and early risk signals. Each metric is derived from verified audit log data, allowing teams to identify deviations, measure progress, and make informed decisions in real time. Alethium’s analytics transform complex trial data into actionable operational insight.

